Abstract
Stress-related variations of fluoride concentration in supernatant saliva and salivary sediment, salivary cortisol, total protein and pH after acute mental stress were assessed. The hypothesis was that stress reactions have no influence on these parameters. Thirty-four male students were distributed into two groups: first received the stress exposure followed by the same protocol two weeks later but without stress exposure, second underwent the protocol without stress exposure followed by the stress exposure two weeks later. The stressor was a public speech followed by tooth brushing. Saliva was collected before, immediately after stress induction and immediately, at 10, 30 and 120 min. after tooth brushing. Cortisol concentrations, total protein, intraoral pH and fluoride content in saliva were measured. The data were analyzed statistically. Salivary sediment was ca 4.33% by weight of whole unstimulated saliva. Fluoride bioavailability was higher in salivary sediment than in supernatant saliva. The weight and fluoride concentration was not altered during 2 hours after stress exposure. After a public speech, the salivary cortisol concentration significantly increased after 20 minutes compared to the baseline. The salivary protein concentration and pH also increased. Public speaking influences protein concentration and salivary pH but does not alter the fluoride concentration of saliva.
Similar content being viewed by others
Introduction
Salivary flow rate, salivary composition, fluoride bioavailability and lifestyle-related factors, such as mental stress, have been recognized as important for oral health1,2,3. Normal whole saliva is composed of water, electrolytes, proteins, hormones and cellular components, such as desquamated mucosal epithelium and microorganisms4,5,6. After centrifugation, two fractions of saliva are separated: supernatant saliva and salivary sediment4,6,7. Salivary sediment consists of organic material, such as oral bacteria, epithelial cells and proteins and inorganic substances, including electrolytes such as fluoride ions5,8. Supernatant saliva is composed of 99% water and a variety of electrolytes, including sodium, potassium, calcium, magnesium, bicarbonate, phosphates7 and proteins9,10.
Usually, salivary composition and saliva's consequent traits undergo fluctuations due to the influence of various factors: daily and seasonal circadian rhythms11, taste and smell12 and short-term acute mental stress13,14. The complex patterns of salivary responsiveness during mental stress are reflected by an increase in total salivary protein concentration13,14,15 and changing cortisol levels16.
Salivary proteins support the ecology of the oral cavity, increase defensive mechanisms in the mouth3 and determine the chemical and physical properties of saliva, such as viscosity17 and lubrication18. Salivary proteins have special functions not only for oral cavity health but also for microorganisms, including nutrition, survival and colonization19,20, as well as for microorganism adhesion and aggregation21. Additionally, salivary proteins and other saliva components that selectively adsorb to bacteria form a “salivary-acquired bacterial pellicle” that likely modifies the adhesive character of the bacterial surface22 and supposedly the whole salivary sediment.
Increased total protein concentration after short-term acute stress changes the chemical properties of saliva, such as the adhesion or lubrication of oral surfaces13.
Fluoride in saliva is essential for the balance between enamel demineralization and remineralization. It is a very reactive anion which can bind easily to positive charged molecules and surfaces. Bound fluoride to the sediment is not reactive. Bioavailability of fluoride in the saliva is defined as the amount of free fluoride ions in the salivary supernatant23. Bioavailable fluoride enhances the mineralization of calcium and phosphate into hydroxyapatite, which remineralizes the crystalline structures of tooth lesions3.
Salivary sediment binds fluoride ions and has its own fluoride clearance kinetics6,8. The distribution of calcium ions between the two salivary fractions was investigated by McGaughey in 197524 and the distribution of fluoride ions was analyzed by Naumova et al. in 20128. It has been shown that the fluoride concentration in the liquid phase of saliva decreases after fluoride application after 30 minutes and is reaching the baseline level after 120 minutes again. In salivary sediment the fluoride concentration is remaining higher8. Fluoride normally is applied with fluoride containing toothpaste twice daily during tooth brushing. Throughout the whole day individuals are exposed to changing physiological conditions which may alter salivary composition. One of these conditions which frequently occurs is mental stress. The relative amounts of salivary sediment and supernatant saliva may change under pathological conditions25 and pathological conditions may thus influence fluoride bioavailability and promote the progression of dental caries26. No data are available in the literature about the relative amounts of salivary sediment and supernatant saliva in stimulated and unstimulated saliva in healthy subjects. It is still not entirely clear how changes in salivary composition after acute mental stress influence saliva's solubility properties and the distribution of inorganic components (electrolytes) in the salivary sediment and supernatant saliva of stimulated and unstimulated saliva in healthy people. Exploration of the changes in salivary content and its properties can help to clarify the mechanisms of fluoride bioavailability and the factors that influence oral health.
The goal of this study was to determine the relative amounts of the two whole saliva fractions: salivary sediment and supernatant saliva and the corresponding fluoride ion distributions after acute mental stress in stimulated and unstimulated saliva, considering the stress-dependent total salivary protein concentration and pH fluctuation in healthy people.
Methods
Ethics statement
The protocol was approved by the ethics committee of Witten/Herdecke University (#39/2009). All experiments were performed in accordance with relevant guidelines and regulations.
Study design
This two-arm cross-over study included 34 healthy male participants (20–30 yrs. old) who were all dental medical or medical students. Verbal and written consent regarding the study design and investigation were obtained before beginning the study. The inclusion criteria were good oral and general health and the exclusion criteria were hormonal medication and endocrinological diseases. The participants were randomly allocated into two groups: The first group (n = 17) received the stress exposure first (subgroup stress 1) followed by the same protocol two weeks later but without the stress exposure (subgroup control 2). The second group (n = 17) underwent the protocol without the stress exposure first (subgroup control 1) followed by the stress exposure two weeks later (subgroup stress 2).
The investigation period was scheduled in autumn between 2:00 and 4:30 p.m. so that cortisol oscillation due to the circadian rhythm could be standardized and minimized16. Baseline values (T0) of unstimulated saliva were collected at 2:00 p.m. for five min. The following stress exposure was achieved by a public speech in front of an audience composed of university lecturers and students and was always performed between 2 p.m. and 2.30 p.m. in the same room of the university27,28. The stress exposure was a question from one of the university lecturers concerning general knowledge. Study subjects were given a two-minute reflection period, after which they performed their two-minute speech. Immediately after stress induction, unstimulated whole saliva samples (T1) were collected for five min. Afterwards, all participants brushed their teeth for three min. with the Bass tooth brushing method29 with an identical toothbrush (ELMEX®, soft, short head “elmex Kariesschutz interX”) using an amine fluoride-containing dentifrice (see below). Tooth brushing served for fluoride application as well as stimulation of saliva secretion. Immediately after tooth brushing, the entire salivary slurry was collected (stimulated whole saliva sample T2). Then, 10 (T3), 30 (T4) and 120 (T5) min. after tooth brushing, additional unstimulated whole saliva samples were collected for five min. each. A similar chronological protocol was performed for the control investigation, only without the public speech.
Oral hygiene product
Amine fluoride (AmF) was administered as a dentifrice (ELMEX®, Gaba, Lörrach, Germany) containing 1400 ppm fluoride from Olaflur®. One gram of dentifrice was weighed and administered onto the toothbrush.
Whole saliva sampling
Stimulated (T2 for three min.) and unstimulated (T0, T1, T3, T4 and T5 for five min. each) whole saliva was collected from each participant into previously weighed 20 ml tubes for the control and stress protocols. Stimulated saliva after tooth brushing was collected by spitting the whole amount of saliva into the prepared tubes in a sitting position. Unstimulated whole saliva sampling was performed in a seated position with open eyes in a silent atmosphere with the head tilted forward while making as few orofacial movements as possible. Visible blood contamination of all whole saliva samples was excluded16.
All samples were frozen and stored at −80°C until cortisol, protein and fluoride assays were performed. Cortisol and fluoride samples were centrifuged before freezing.
Amounts of whole saliva and salivary sediment
The weight of whole saliva was measured in the collected samples using a precision scale (Sartorius, CP Series CPA 1245, Göttingen, Germany) by calculating the difference between the weight of the empty tube and the tube containing the saliva sample. Saliva consists of more than 99% water7; therefore, the saliva was presumed to have the same density as water (1 g ≙ 1 ml)8,14.
Microcentrifuge tubes were weighed, filled with 1.5 ml of whole saliva and centrifuged. After centrifugation of the sample for 10 min. at 3000 × g, the supernatant saliva was completely removed from the microcentrifuge tubes using a pipette. The residual salivary sediment within the microcentrifuge tube samples was weighed again and calculated as a percentage of whole saliva.
Fluoride determination in supernatant saliva
Whole saliva samples were centrifuged for 10 min. at 3000 × g in microcentrifuge tubes. The supernatant salivary fluoride analysis was performed after mixing 1 ml of saliva with 1 ml of TISAB II buffer solution (Thermo Scientific, Waltham, MA, USA) using a fluoride-sensitive electrode (96-09 Orion, Thermo Electron, Beverly, MA, USA). All supernatant saliva samples were measured three times and the mean value was used for further statistical analysis. Direct calibration and incremental techniques (the method of known addition for low ionic strength samples with a fluoride concentration less than 0.38 ppm) were standardized and performed as analytical techniques. Afterward, a series of prepared fluoride standards was used for direct calibration: 0.4, 4.0, 40 and 400 ppm.
Fluoride determination in salivary sediment
Salivary sediment samples for fluoride determination were removed with the pipette from the microcentrifuge tubes, replaced in a previously weighted 4 ml tubes, weighed and then mixed with 500 µl of TISAB II buffer by vortexing ca. 10 seconds until a homogeneous solution was achieved. The fluoride content of the salivary sediment was analyzed as described above. The dilution of the salivary sediment with TISAB II has been taken into account for the calculated fluoride concentration.
Stress and salivary cortisol evaluation
The stress reaction was determined as previously described14 by analyzing saliva cortisol concentration. A significant increase in salivary cortisol concentration after the public speech compared to baseline values indicated a positive stress reaction. A commercial enzyme-linked immunosorbent assay (Cortisol ELISA, Immunoassay RE 52611 from IBL International, Hamburg, Germany) was used to determine the amount of salivary cortisol in supernatant saliva. There was cross-reactivity of the anti-cortisol antibody with other relevant steroids: 7.0% 11-deoxycortisol, 4.2% cortisone, 1.4% corticosterone, 0.35% progesterone and <0.01% testosterone, estrone, estradiol and estriol. The intra- and interassay variance averaged 4.8% and 5.9%, respectively.
Total salivary protein concentration
A total of 100 μl of saliva was centrifuged at 3000 × g. Total protein contents were photometrically measured from the supernatants according to the Bradford method at 595 nm30 and 0.1, 0.3, 0.5 and 0.7 mg/ml solutions of bovine serum albumin served as standards.
Salivary pH
An intraoral salivary pH measurement was performed after each saliva collection. The study participants were asked to collect saliva in the area of the vestibulum oris of the lower jaw. The pH was measured with a Beetrode (World Precision of Instruments, WPI) and an Orion 4 stars series measuring device (sn 007352 Thermo Electron Corporation, Beverly, USA). After previous two-point calibration of the Beetrode with standard solutions of pH between 5 and 7, as well as between 7 and 10, the lower lip was held with a wooden spatula and the tip of the electrode was placed into the saliva in the vestibule for 30 seconds until stabilization of the measured salivary pH value on the monitor and visual confirmation from the measuring device.
Statistical methods
The obtained data were processed with the Statistical Package for Social Sciences (SPSS 18.0, Chicago, III, USA). The carry-over effect was determined by comparing the data of stress 1 plus control 2 with control 1 plus stress 2 using the non-parametric Wilcoxon-Mann-Whitney test. Data on fluoride bioavailability in supernatant saliva and salivary sediment, salivary secretion rate, intraoral salivary pH and salivary cortisol concentration between time points T0, T1, T2, T3, T4 and T5 were analyzed with the nonparametric sign test for related variables. Five tests were applied to the data for the time intervals and the α-adjustment after Bonferroni resulted in a p-value of p < 0.01 for those tests. To compare the results for fluoride bioavailability in supernatant saliva, the salivary sediment and salivary flow rate between the control and stress group curves were plotted for every individual for all time intervals and the area under the curve (AUC) was calculated and compared. The non-parametric Wilcoxon-Mann-Whitney test for independent variables was applied. The level of significance for the comparison between the two groups was p < 0.05.
Results
Carry-over effect
A positive carry-over effect between the groups stress 1 – control 2 and control 1– stress 2 was found for the salivary cortisol concentration and fluoride concentration in salivary sediment. Therefore, the cortisol and fluoride concentrations in salivary sediment and supernatant saliva were calculated separately.
No carry-over effect was determined for fluoride concentration in supernatant saliva, salivary total protein, pH and amount of salivary sediment and supernatant saliva. Therefore, the statistical analysis for these parameters was performed by pooling both control subgroups and both stress subgroups data (with the exception of supernatant saliva).
Amount of saliva and salivary sediment
There was a statistically significant increase of the total salivary weight (in g) immediately after tooth brushing (T2) (stimulated saliva) compared to T0 in all subgroups (Tables 1 and 2, Fig. 1a). The same was found for supernatant saliva (Table 1 and 2, Fig. 1b). There was also a significant increase of the total weight of salivary sediment except in the subgroup control 2 (Table 1 and 2, Fig. 1c). The mean (±SD) relative amount of salivary sediment in healthy subjects was 4.33 ± 3.21% of the whole saliva weight. The relative amount of salivary sediment did not significantly change after acute mental stress during the entire test period.
Fluoride bioavailability in supernatant saliva
The fluoride concentration in supernatant saliva in all subgroups at baseline (T0) was between 0.029 and 0.048 ppm with no statistically significant difference between the subgroups. The highest fluoride concentration was reached immediately after tooth brushing (T2); the fluoride concentration decreased within 30 min. (T4), but remained higher than that at baseline. After 2 hours (T5), fluoride bioavailability in supernatant saliva returned to baseline values (Table 3 and 4, Fig. 2).
Comparison of the AUC calculations using the non-parametric Wilcoxon-Mann-Whitney test revealed no statistically significant differences between the subgroups stress 1 and control 2 (p = 0.956), control 1 and stress 2 (p = 0.521).
Fluoride bioavailability in salivary sediment
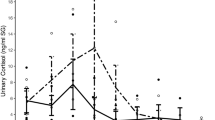
At baseline (T0), the salivary sediment fluoride concentration in the control subgroups was between 0.796 and 3.15 ppm. The highest fluoride concentration was reached immediately after tooth brushing (T2), but the fluoride concentration decreased within 30 min. (T4). After 2 hours (T5), the fluoride concentration was two times higher than that measured at T0; however, this difference did not show statistical significance in the stress subgroups (Table 3 and 4, Fig. 3).
Boxplot graphics of the distribution of the measured fluoride concentrations in salivary sediment.
The highest fluoride concentration was reached immediately after fluoride application (T2). The fluoride concentration 120 (T5) min. after tooth brushing was higher than at the baseline (T0) in all subgroups. In the stress subgroups at T5 was a strong variation.
There were no significant differences between the salivary sediment fluoride concentrations in the subgroups stress 1 and control 2 (p = 0.072), control 1 and stress 2 (p = 0.669) after performing the non-parametric Wilcoxon-Mann-Whitney test.
Ratio of fluoride bioavailability and weight between salivary sediment and supernatant saliva
The ratio between the fluoride bioavailability in salivary sediment and supernatant saliva (F ratio) in the control 1, control 2 and stress 2 subgroups changed significantly between T2 and T3 (Table 5), whereas no significant differences were found at any time interval in the stress 2 subgroup.
The comparison of the F ratio and weight ratio between the subgroups stress 1 – control 2 and control 1 – stress 2 revealed no significant differences.
Salivary cortisol concentration
The salivary cortisol concentration in the subgroups stress 1 and control 2 at baseline (T0) was 7.23 ± 4.79 and 3.91 ± 1.97 nmol/l and did not differ significantly. In the subgroup stress 1, the cortisol level increased 20 min. after stress exposure (T3) to a level of 14.73 ± 8.99 nmol/l and was significantly (p = 0.002) higher than in the subgroup control 2. In the subgroups control 1 and stress 2, no significant differences in the salivary cortisol levels were found (Fig. 4).
Salivary total protein content
The baseline salivary protein content in the control subgroups was 0.81 ± 0.31 mg/ml. The salivary protein content decreased to 0.63 ± 0.35 mg/ml at T1, with little change at T2 (0.61 ± 0.34). At T3, the protein content increased slightly to 0.69 ± 0.27 mg/ml. Throughout the remainder of the experiment, the protein concentration further increased from 0.71 ± 0.38 mg/ml at T4 to 0.75 ± 0.54 mg/ml at T5.
In the stress subgroups at baseline (T0), a protein concentration of 0.92 mg/ml was determined. After stress exposure (T1), an increase in the protein content to 1.02 ± 0.44 mg/ml was observed. A similar concentration of 1.09 ± 0.77 mg/ml was measured at T2. The protein concentration continuously decreased from 0.98 ± 0.47 mg/ml at T3 to 0.79 ± 0.34 mg/ml at T4 and finally to 0.74 ± 0.40 mg/ml at T5 (Fig. 5).
A comparison between the control and stress groups using the non-parametric Wilcoxon-Mann-Whitney test showed that the protein concentration at T1 in the stress subgroups was significantly higher than that in the control subgroups.
Salivary pH
The intraorally measured salivary pH value in the control subgroups during the two hour investigation period showed no significant changes: at baseline, the value was (T0) 7.6 ± 0.5; at T1, the value was 7.5 ± 0.4 (p = 0.774); immediately after tooth brushing (T2), the pH was 7.6 ± 0.3 (p = 1.0); after 10 min. (T3), the pH was 7.7 ± 0.3 (p = 0.774); after 30 min. (T4), the pH was 7.7 ± 0.4 (p = 0.774); and after 2 hours (T5), the pH was 7.8 ± 0.6 (p = 0.065). The pH value in the stress subgroups showed no significant changes from time points T0 to T5. At time intervals T0 to T3, the pH values were as follows: T0, 7.8 ± 0.60; T1, 7.5 ± 0.7 (p = 0.424); T2, 7.8 ± 0.7 (p = 1.0); T3, 8.3 ± 1.1 (p = 0.013); and T4, 8.4 ± 1.0 (p = 0.013). At T5, the differences in the pH compared to T0 were significant (T5: 8.2 ± 0.7; p < 0.001) (Fig. 6). By applying the non-parametric Wilcoxon-Mann-Whitney test, no significant differences in salivary pH values between the stress and control subgroups at T0 to T3 were revealed. At T4 and T5, the differences between the control and the stress subgroups were significant (T4: p = 0.041; T5: p = 0.037).
Discussion
Saliva is essential for maintaining oral health. Fluoride is an important component in this rather complex system. Saliva consists of two fractions with different chemo-physical properties: supernatant saliva and salivary sediment.
Investigations that address the distribution of fluoride in salivary sediment and supernatant saliva are scarce4,6,7,8,27,31. The majority of publications about whole saliva content deal with supernatant saliva. Some studies have shown that AmF binds to plaque32 and reduces the viability of plaque bacteria33. There are no data about the weight and the fluoride distribution between the two fractions of whole saliva under different psychological conditions in healthy people.
The data from the present study showed that public speaking induces stress reactions that include a significant increase in the salivary cortisol concentration, confirming a previous investigation14,27 The limitation of the investigated cohort to healthy male participants of the same age group helped to exclude age34, health state- and gender-dependent35,36,37 salivary cortisol fluctuations and to minimize cortisol analysis misinterpretation.
The total protein concentration in the saliva of the stress subgroups significantly increased, immediately after stress exposure (T1), confirming the results of investigation by Bosch et al., 199613. The increase in the total protein concentration after stress exposure may be due to sympathetic activation during stress as the sympathetic innervation of the salivary glands controls protein secretion12,38. Recent studies14,27 showed that the salivary flow rate does not change during stress responses. The intraoral salivary pH at 30 min. and up to 120 min. after stress exposure was significantly more alkaline compared to the control group. There are some indications that cortisol can affect acid-base transporters. In the kidney proximal tubule, increased expression of the NaHCO3 cotransporter can be induced by applying hydrocortisone39. Additionally, increased adrenaline increases renal NaHCO340. Because the NaHCO3 cotransporter is important for the secretion of bicarbonate into the saliva, this mechanism could be a possible explanation for the observed changes in pH. Dietary induced salivary pH changes41 were minimized by abstaining from any food uptake during the investigation period.
In the present study, it was found that the amount of salivary sediment in healthy subjects was approximately 4.33% of whole unstimulated saliva; this amount did not change significantly throughout the entire test period. However, at time point T2 a significant increase in the total weight of salivary sediment was measured except for the group control 2. Salivary sediment is composed of desquamated cells and bacteria, which may bind the inorganic and organic constituents of supernatant saliva. It can be hypothesized that salivary sediment is involved in the regulation of dissolved constituents within supernatant saliva. In this context, salivary sediment may play an important role in maintaining oral health.
It was also shown that the fluoride distribution between the salivary sediment and supernatant saliva is unequal and varies considerably. After tooth brushing, the increase in fluoride in sediment was higher than that in supernatant saliva. These results are in accordance with those of a previous study8. The data from the present investigation suggest that fluoride binds to the salivary sediment to a limited extent as the ratio between the fluoride concentrations in sediment and supernatant saliva increases with increasing fluoride content immediately after tooth brushing. The fact that salivary sediment always showed higher fluoride concentrations than supernatant saliva indicates that AmF is associated with the organic constituents of salivary sediment. Therefore, the salivary sediment serves as a reservoir for fluoride ion bioavailability.
Regarding fluoride bioavailability in supernatant saliva, a sudden decrease by T1 compared to baseline values in the control subgroups was observed. A possible explanation is the spontaneous redistribution of fluoride ions between supernatant saliva and salivary sediment, considering the increase in fluoride bioavailability in salivary sediment. The fact that the increase in fluoride bioavailability in salivary sediment at T5 was not significant compared to the baseline value can be explained by the major difference between the absolute values of fluoride bioavailability in supernatant saliva and salivary sediment.
It can therefore be assumed that the distribution of AmF between the salivary sediment and supernatant saliva is influenced by the binding behavior of AmF to organic constituents. AmF may bind not only to bacterial surfaces but also to the cell surfaces of salivary sediment, thus increasing the capacity for fluoride retention.
After stress exposure, the fluoride concentration in the salivary sediment was always higher (T1-T5) than that of the control subgroups but without statistical significance. Therefore, there is only a tendency towards a higher fluoride concentration in the salivary sediment after stress exposure. These considerable changes in the distribution between the salivary sediment and supernatant saliva may be due to fluoride retention in the salivary sediment. However, a clear effect of stress reactions on the bioavailability of fluoride in supernatant saliva could not be shown, confirming the results of a previous investigation27.
The present study has the following limitations: (1) the test subjects were only men, which excluded gender-dependent cortisol fluctuation34,35,36 and (2) all of the men belonged to the same age group.
The results of this investigation showed that acute mental stress during a 120-minute period after stress exposure does not influence fluoride bioavailability in either salivary fraction. The null hypothesis was therefore supported. However, the results also supported the hypothesis that salivary sediment serves as a reservoir for fluoride ions to maintain fluoride bioavailability in supernatant saliva.
Conclusions
The amount of salivary sediment in healthy people was approximately 4.33% by weight of whole unstimulated and stimulated after tooth brushing saliva. The salivary protein increase and pH changes after acute mental stress showed no influence on the salivary sediment amount within two hours after stress. Fluoride bioavailability in the supernatant saliva and salivary sediment is not affected by acute mental stress. Further investigations are necessary to appraise the role of salivary sediment in maintaining salivary composition homeostasis. Further investigations should also address the feeling of dry mouth during acute mental stress, which is a subjective indication of alterations in salivary tribological properties and possibly other chemo-physical salivary properties, such as changing solubility.
References
Edgar, W. M., Higham, S. M. & Manning, R. H. Saliva stimulation and caries prevention. Adv Dent Res 8, 239–245 (1994).
Lagerlof, F. & Oliveby, A. Caries-protective factors in saliva. Adv Dent Res 8, 229–238 (1994).
Selwitz, R. H., Ismail, A. I. & Pitts, N. B. Dental caries. Lancet 369, 51–59, (2007).
Helmerhorst, E. J., Traboulsi, G., Salih, E. & Oppenheim, F. G. Mass spectrometric identification of key proteolytic cleavage sites in statherin affecting mineral homeostasis and bacterial binding domains. J Proteome Res 9, 5413–5421 (2010).
Ryan, C. S. & Kleinberg, I. A comparative study of glucose and galactose uptake in pure cultures of human oral bacteria, salivary sediment and dental plaque. Arch Oral Biol 40, 743–752 (1995).
Schipper, R. G., Silletti, E. & Vingerhoeds, M. H. Saliva as research material: biochemical, physicochemical and practical aspects. Arch Oral Biol 52, 1114–1135 (2007).
Humphrey, S. P. & Williamson, R. T. A review of saliva: normal composition, flow and function. J Prosthet Dent 85, 162–169 (2001).
Naumova, E. A. et al. Kinetics of fluoride bioavailability in supernatant saliva and salivary sediment. Arch Oral Biol 57, 870–876 (2012c).
Al Kawas, S., Rahim, Z. H. & Ferguson, D. B. Potential uses of human salivary protein and peptide analysis in the diagnosis of disease. Arch Oral Biol 57, 1–9 (2012).
Morzel, M. et al. Saliva electrophoretic protein profiles in infants: changes with age and impact of teeth eruption and diet transition. Arch Oral Biol 56, 634–642 (2011).
Hansen, A. M., Garde, A. H. & Persson, R. Sources of biological and methodological variation in salivary cortisol and their impact on measurement among healthy adults: a review. Scand J Clin Lab Invest 68, 448–458 (2008).
Carpenter, G. H. The secretion, components and properties of saliva. Annu Rev Food Sci Technol 4, 267–276 (2013).
Bosch, J. A. et al. Psychological stress as a determinant of protein levels and salivary-induced aggregation of Streptococcus gordonii in human whole saliva. Psychosom Med 58, 374–382 (1996).
Naumova, E. A. et al. Acute short-term mental stress does not influence salivary flow rate dynamics. PloS one 7, e51323 (2012b).
Bosch, J. A., de Geus, E. J., Veerman, E. C., Hoogstraten, J. & Nieuw Amerongen, A. V. Innate secretory immunity in response to laboratory stressors that evoke distinct patterns of cardiac autonomic activity. Psychosom Med 65, 245–258 (2003).
Tornhage, C. J. Salivary cortisol for assessment of hypothalamic-pituitary-adrenal axis function. Neuroimmunomodulation 16, 284–289 (2009).
Roberts, A. D. Role of electrical repulsive forces in synovial fluid. Nature 231, 434–436 (1971).
Douglas, W. H. et al. Statherin: a major boundary lubricant of human saliva. Biochem Biophys Res Commun 180, 91–97 (1991).
Kindblom, C., Davies, J. R., Herzberg, M. C., Svensater, G. & Wickstrom, C. Salivary proteins promote proteolytic activity in Streptococcus mitis biovar 2 and Streptococcus mutans. Mol Oral Microbiol 27, 362–372, 10.1111/j.2041-1014.2012.00650.x (2012).
Wickstrom, C. & Svensater, G. Salivary gel-forming mucin MUC5B--a nutrient for dental plaque bacteria. Oral Microbiol Immunol 23, 177–182 (2008).
Prakobphol, A. et al. Salivary agglutinin, which binds Streptococcus mutans and Helicobacter pylori, is the lung scavenger receptor cysteine-rich protein gp-340. J Biol Chem 275, 39860–39866 (2000).
Scannapieco, F. A. Saliva-bacterium interactions in oral microbial ecology. Crit Rev Oral Biol Med 5, 203–248 (1994).
Bánóczy, J., Rugg-Gunn, A. & Woodward, W. Milk fluoridation for the prevention of dental caries. Acta Med Acad 42, 156–167 (2013).
McGaughey, C., Campbell, J. E., Pazo, C. & Stowell, E. C. Relations between early dental calculus production and calcium and phosphate parameters of salivary fractions. J Periodontol 46, 681–684 (1975).
Chambers, M. S. et al. Salivary flow rates measured during radiation therapy in head and neck cancer patients: a pilot study assessing salivary sediment formation. J Prosthet Dent 100, 142–146 (2008).
Chambers, M. S. et al. Clinical evaluation of the intraoral fluoride releasing system in radiation-induced xerostomic subjects. Part 1: Fluorides. Oral Oncol 42, 934–945 (2006).
Naumova, E. A. et al. Fluoride bioavailability in saliva during acute psychological stress. Centr Eur J Med 7, 481–489 (2012d).
Pawlak, C. R. et al. Patients with systemic lupus erythematosus differ from healthy controls in their immunological response to acute psychological stress. Brain Behav Immun 13, 287–302, 10.1006/brbi.1999.0553 (1999).
Bass, C. C. An effective method of personal oral hygiene; part II. J La Sate Med Soc 106, 100–112 (1954).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72, 248–254 (1976).
Denepitiya, L. & Kleinberg, I. A comparison of the microbial compositions of pooled human dental plaque and salivary sediment. Arch Oral Biol 27, 739–745 (1982).
Naumova, E. A. et al. Fluoride bioavailability in saliva and plaque. BMC Oral Health 12, 3 (2012a).
van der Mei, H. C., Engels, E., de Vries, J. & Busscher, H. J. Effects of amine fluoride on biofilm growth and salivary pellicles. Caries Res 42, 19–27 (2008).
Jessop, D. S. & Turner-Cobb, J. M. Measurement and meaning of salivary cortisol: a focus on health and disease in children. Stress 11, 1–14 (2008).
Kajantie, E. & Phillips, D. I. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 31, 151–178 (2006).
Kudielka, B. M. & Kirschbaum, C. Sex differences in HPA axis responses to stress: a review. Biol Psychol 69, 113–132, 10.1016/j.biopsycho.2004.11.009 (2005).
Young, E. A. & Altemus, M. Puberty, ovarian steroids and stress. Ann N Y Acad Sci 1021, 124–133 (2004).
Carpenter, G. H., Garrett, J. R., Hartley, R. H. & Proctor, G. B. The influence of nerves on the secretion of immunoglobulin A into submandibular saliva in rats. J Physiol 512 (Pt 2), 567–573 (1998).
Ruiz, O. S., Wang, L. J., Pahlavan, P. & Arruda, J. A. Regulation of renal Na-HCO3 cotransporter: III. Presence and modulation by glucocorticoids in primary cultures of the proximal tubule. Kidney Int 47, 1669–1676 (1995).
Sonalker, P. A., Tofovic, S. P., Bastacky, S. I. & Jackson, E. K. Chronic noradrenaline increases renal expression of NHE-3, NBC-1, BSC-1 and aquaporin-2. Clin Exp Pharmacol Physiol 35, 594–600 (2008).
Fadel, H. T. et al. Profiles of dental caries and periodontal disease in individuals with or without psoriasis. J Periodontol 84, 477–485 (2013).
Acknowledgements
The authors would like to thank Prof. Frank Thévenod (Institute of Physiology, Pathophysiology and Toxicology, Witten/Herdecke University) for valuable discussions, Mrs. Susanne Haussmann (School of Dentistry) for her technical assistance regarding the fluoride measurements and GABA international for providing elmex Kariesschutz interX toothbrushes and ELMEX® toothpaste. This study was supported by Deutsche Gesellschaft für Zahnerhaltung (DGZ).
Author information
Authors and Affiliations
Contributions
E.A.N. calculated the statistics wrote the manuscript, T.S. carried our stress experiments, fluoride determination in supernatant saliva, C.B. fluoride determination in salivary sediment, P.A.K. protein determination, W.K.L. supervision of protein determination and analysis of the results, S.Z. manuscript correction, project planning, W.H.A. planning of the whole project, supervision and final manuscript preparation.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Naumova, E., Sandulescu, T., Bochnig, C. et al. Dynamic changes in saliva after acute mental stress. Sci Rep 4, 4884 (2014). https://doi.org/10.1038/srep04884
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04884
This article is cited by
-
Randomized investigation of the bioavailability of fluoride in saliva after administration of sodium fluoride, amine fluoride and fluoride containing bioactive glass dentifrices
BMC Oral Health (2019)
-
Investigating the association between stress, saliva and dental caries: a scoping review
BMC Oral Health (2018)
-
High-sensitivity ion detection at low voltages with current-driven organic electrochemical transistors
Nature Communications (2018)
-
Parallel study about the effects of psychotherapy on patients with dental phobia determined by anxiety scores and saliva secretion and composition
BMC Oral Health (2017)
-
Dynamics of Fluoride Bioavailability in the Biofilms of Different Oral Surfaces after Amine Fluoride and Sodium Fluoride Application
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.